Sodium Acetate Towers
Materials




Instructions

Reach into your kit & pull out the bag that says Experiment 15. Grab out the bag with the Petri dishes and keep everything else in the bag for now.

Take out the Petri dishes but be sure to keep them closed. Move the white mat to the side, we don’t need it for this experiment.

Next, take out the four little bottles. They contain sodium acetate in water. Pick out two bottles of liquid and place them in front of you. Move the rest of the bottles that are solid to the side.
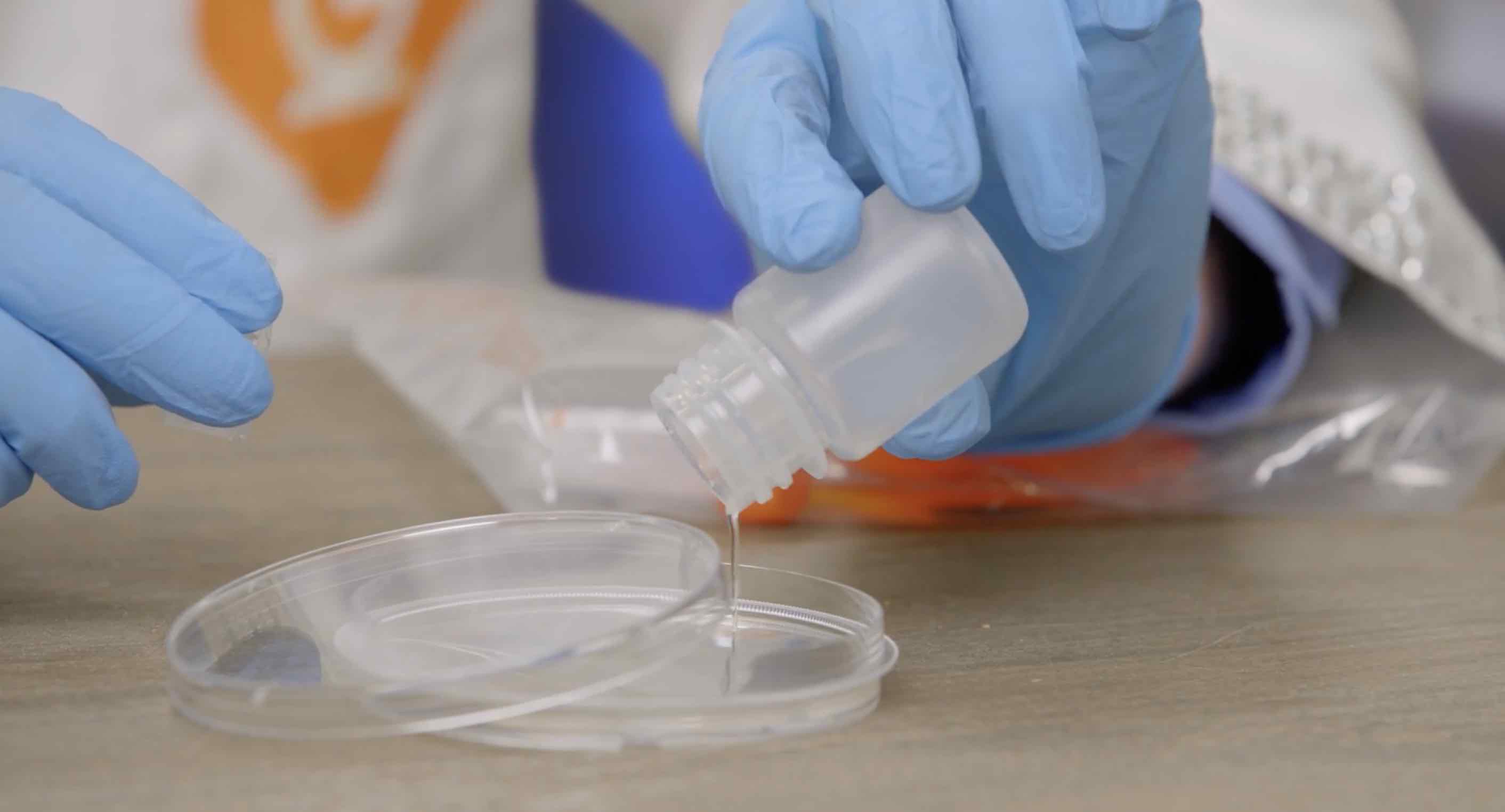
Take the lid off of one of the Petri dishes and pour one of the bottles of liquid into it. Then put the lid back on the Petri dish.
Take your tube of Sodium Acetate Crystals and gently open it. Grab a pinch of crystals and drop them into the center of the liquid in the dish.

Next grab another bottle of liquid and gently uncap. Slowly pour the liquid on top of the solid crystals to grow them even more.

If you have an extra bottle of liquid, that’s a bonus and you can use it to continue building your crystal tower.

You have an extra petri dish so if you have another bottle of liquid you can do it again. Remember, if your bottles are solid, ask an adult to boil them in water for about 10 minutes to turn them back into a liquid.
How It Works
Since this liquid is so sensitive to dust and other particles, it's possible that your reaction happened differently than Dr. Jeff's, and that's okay too. Any kind of dust or seed crystal could set it off, and that's what is so neat about it.
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
We use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More











