Rainbow Electrolysis
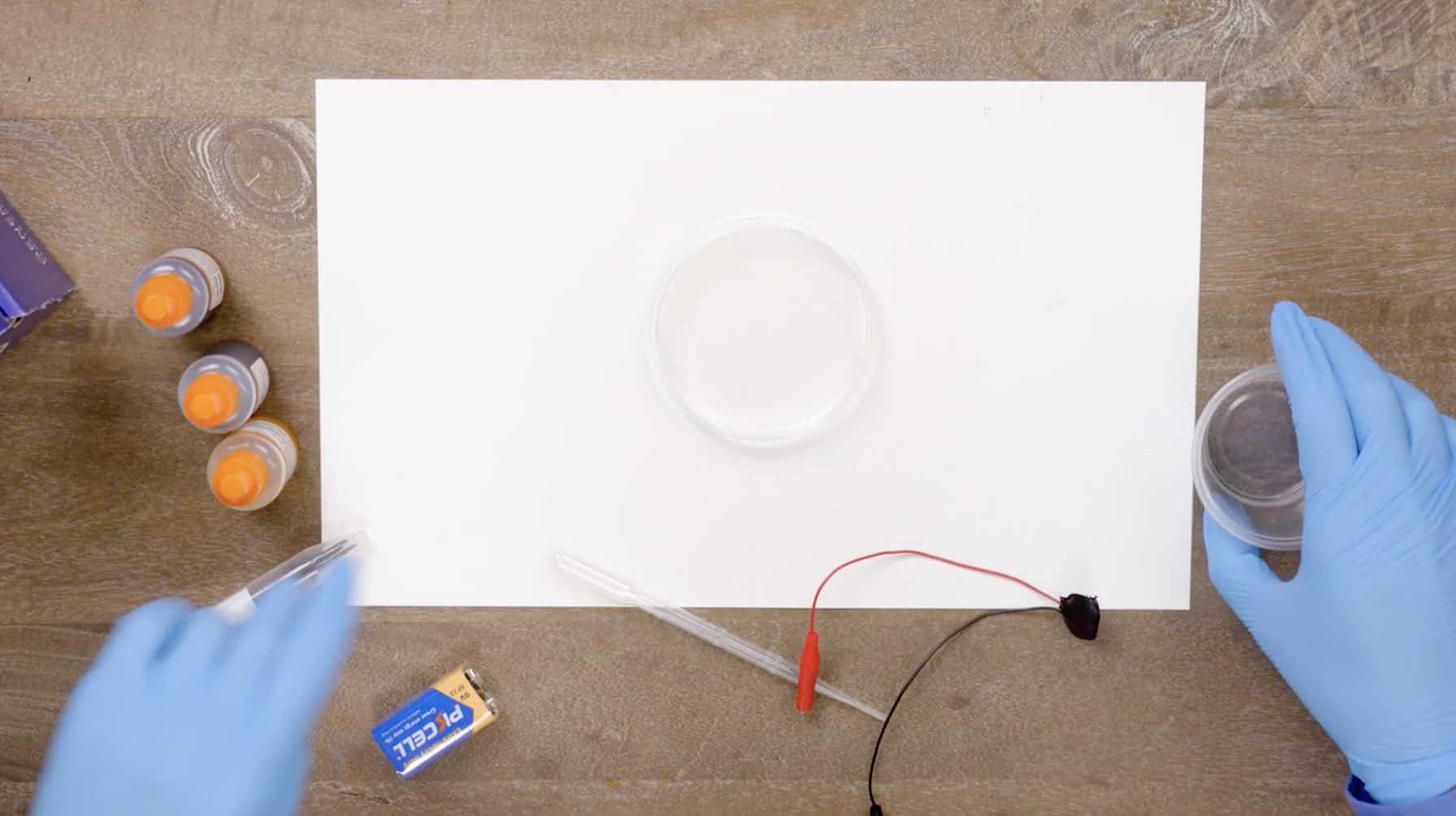
Materials









Instructions

Pull out the bag with the battery in it. Place everything around the white mat and put the bag back in your box.
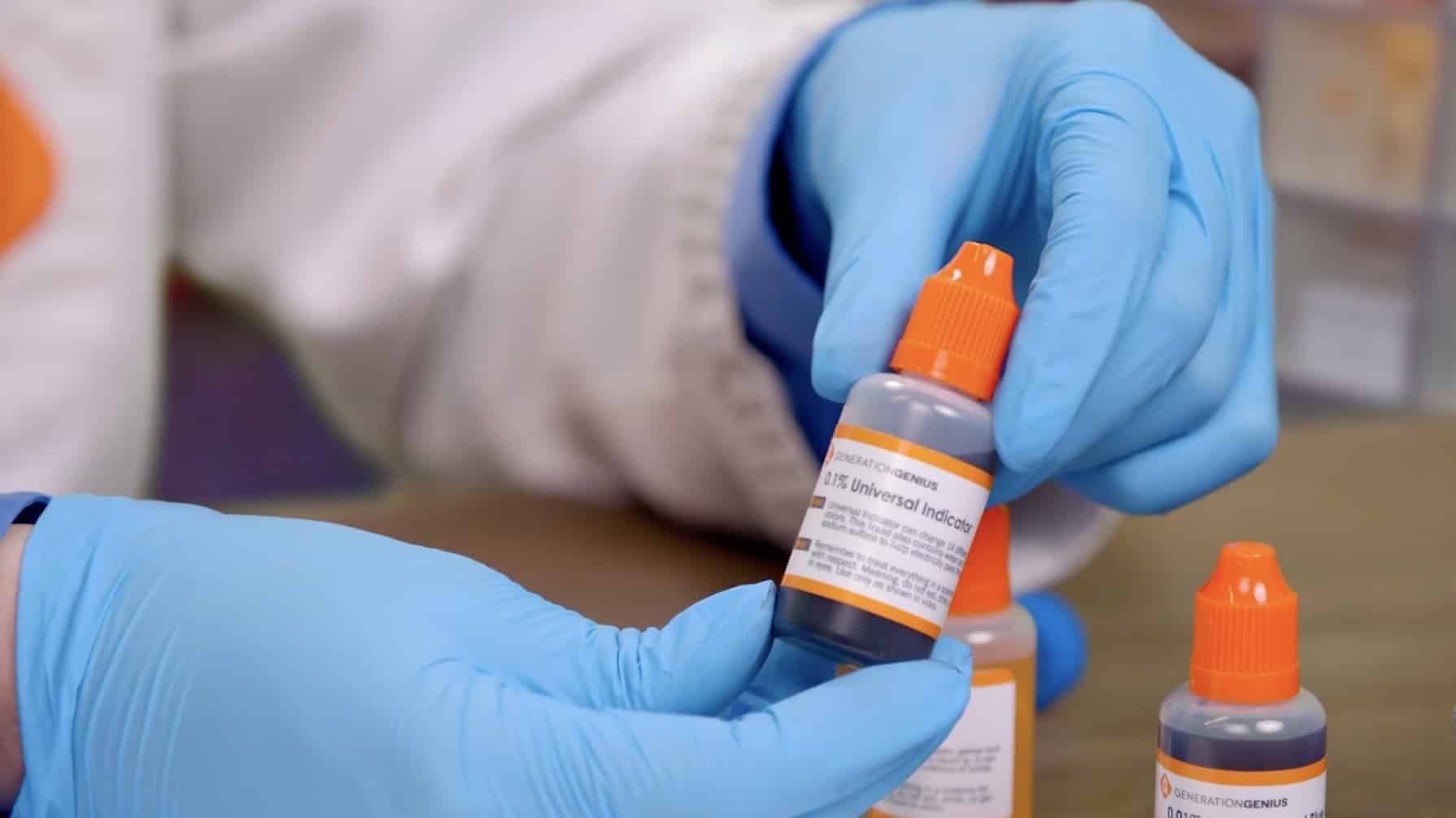
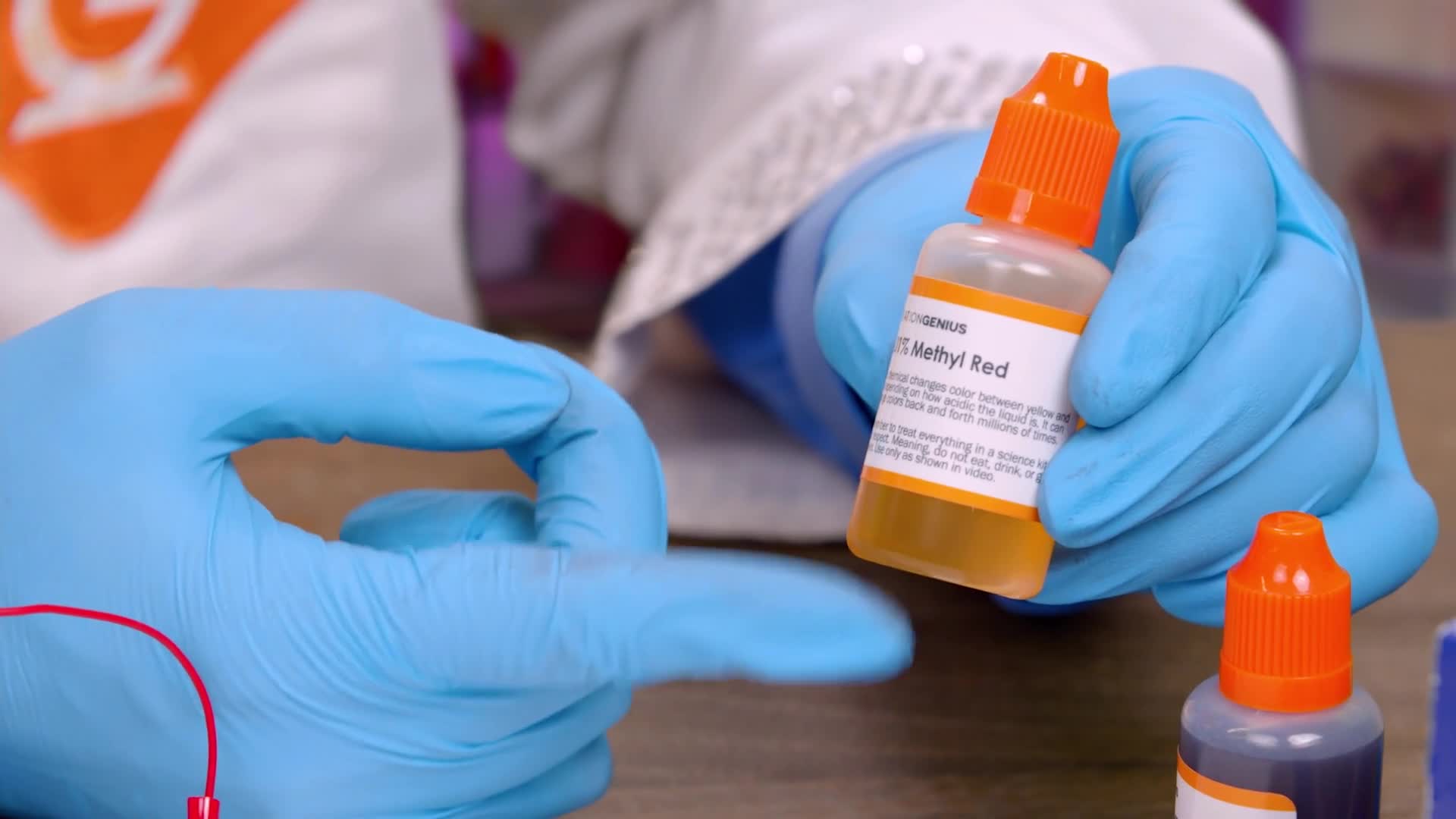
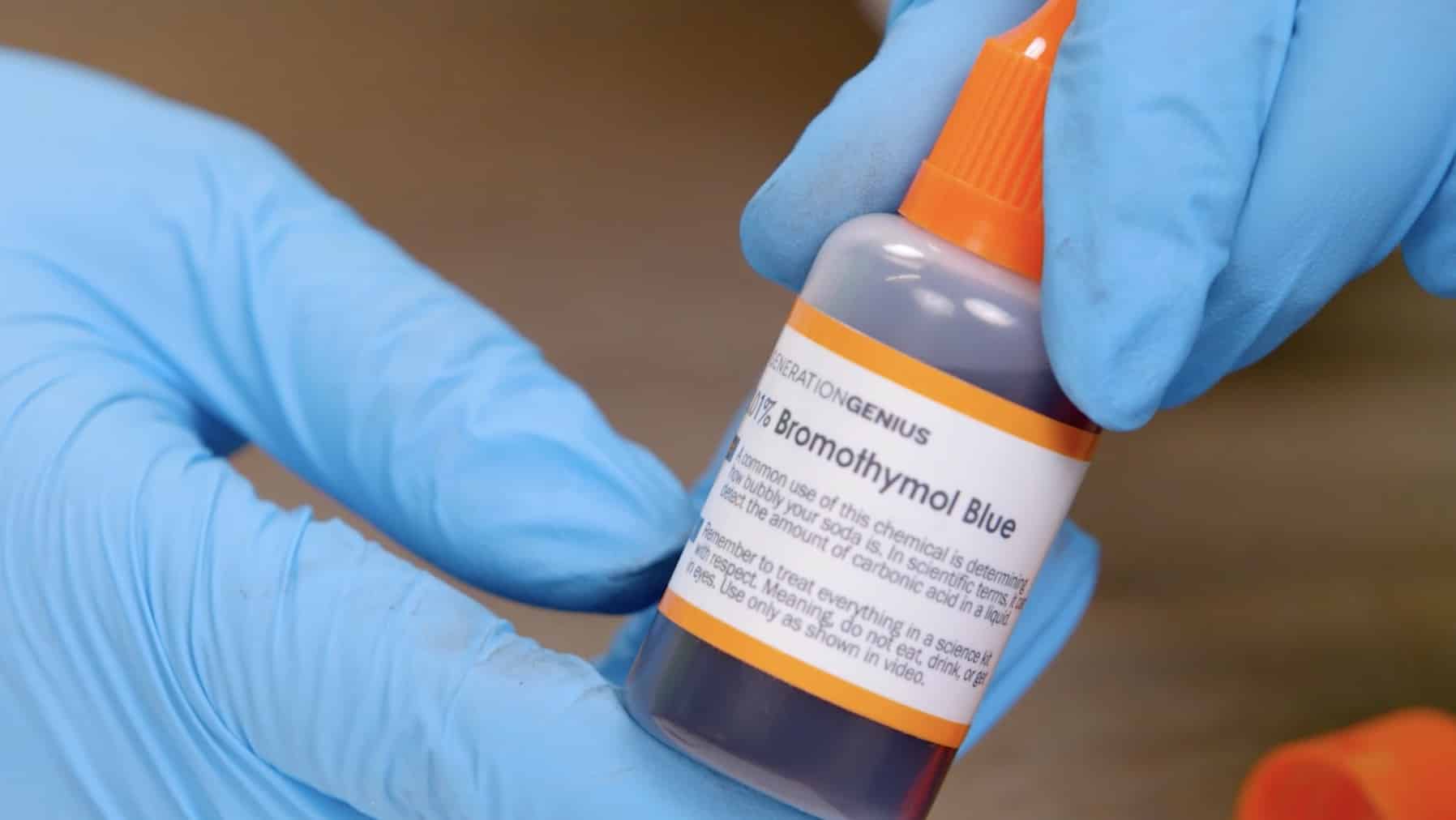
Grab the bottle that says Universal Indicator. To open, simply push down on the top and turn.

Squeeze some of the liquid into the petri dish, but not all of it. Leave about a quarter of the liquid in the bottle.
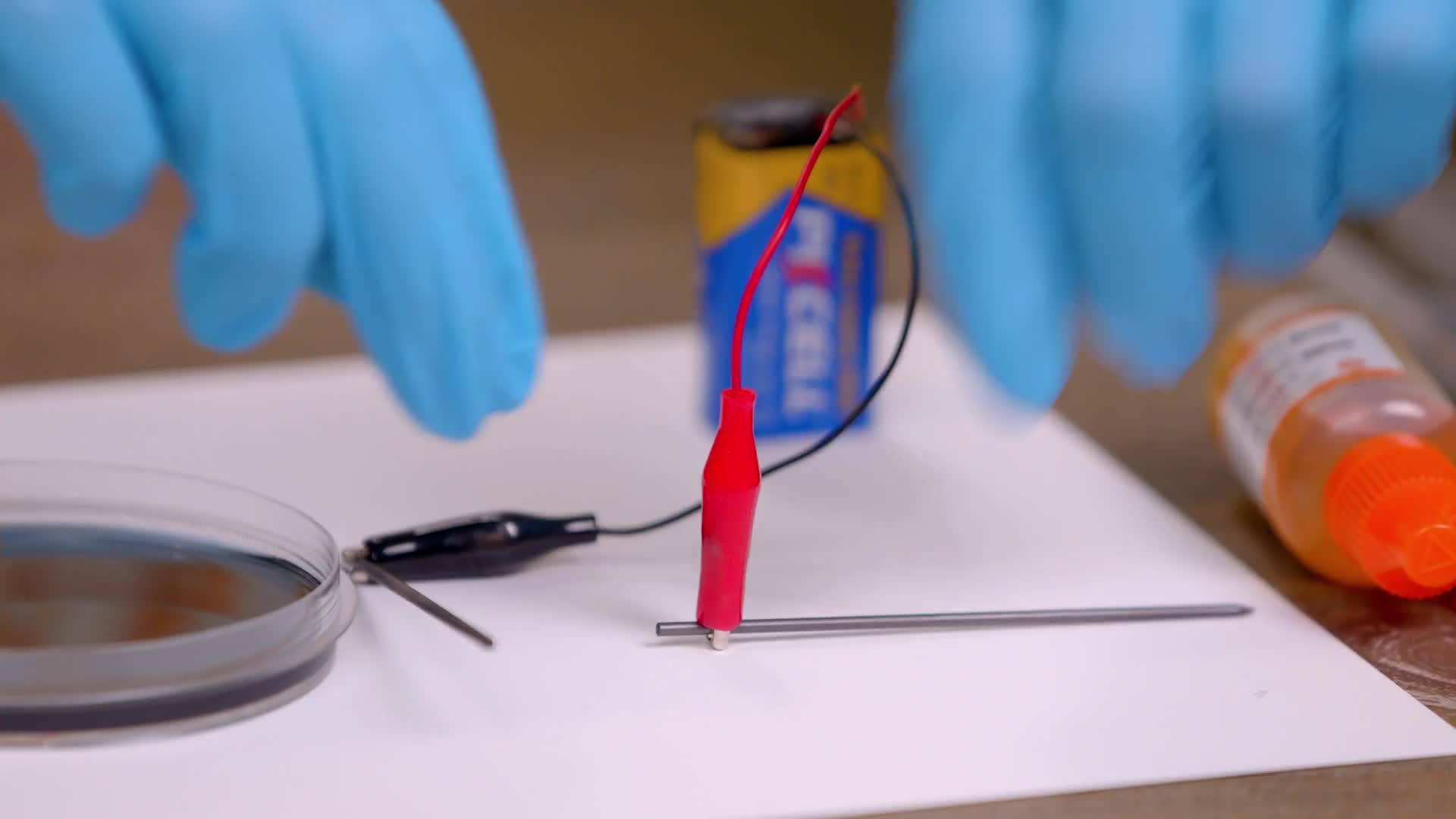
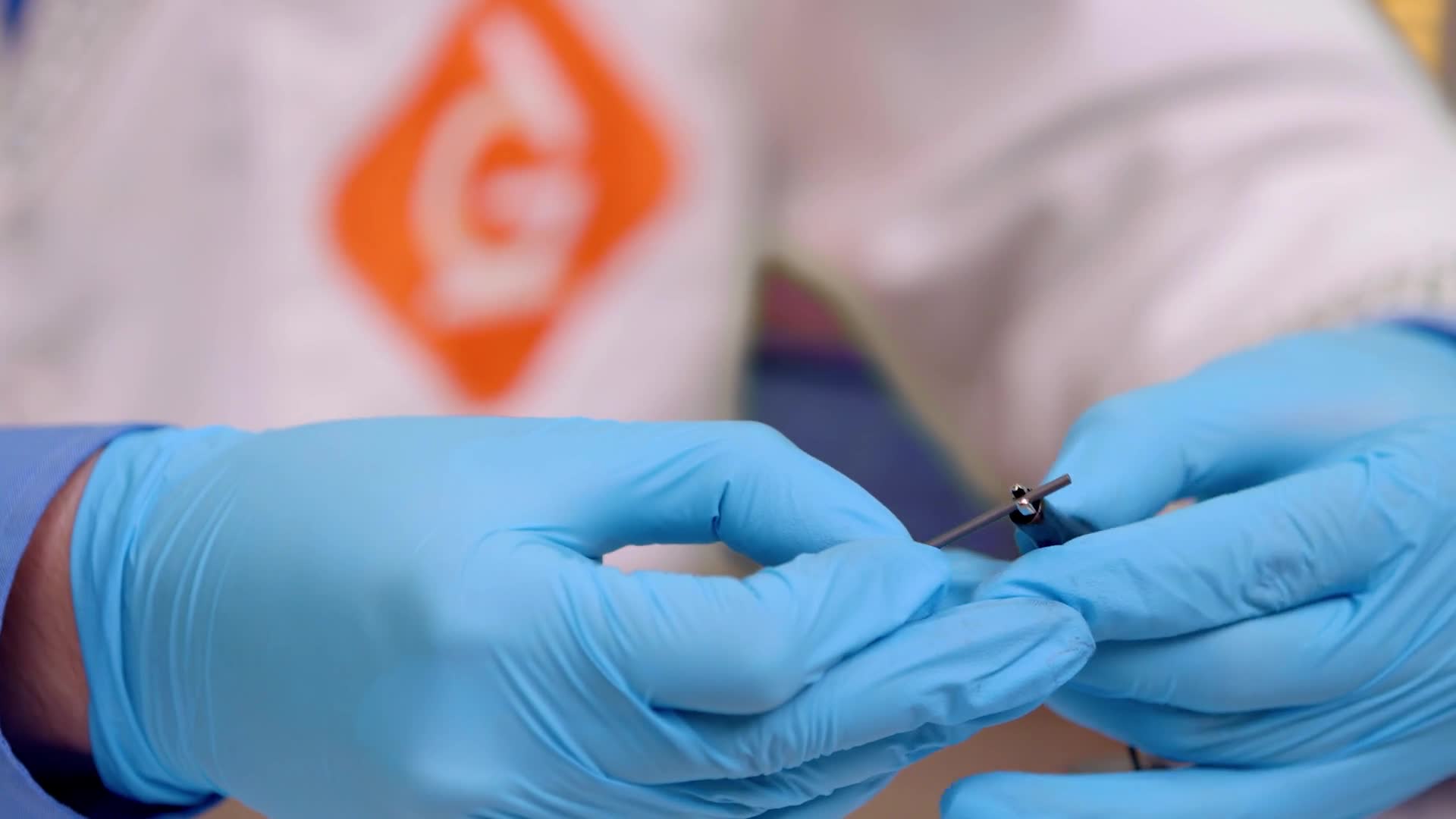
Attach the other connector to another stick of lead. You have extras just in case one breaks.

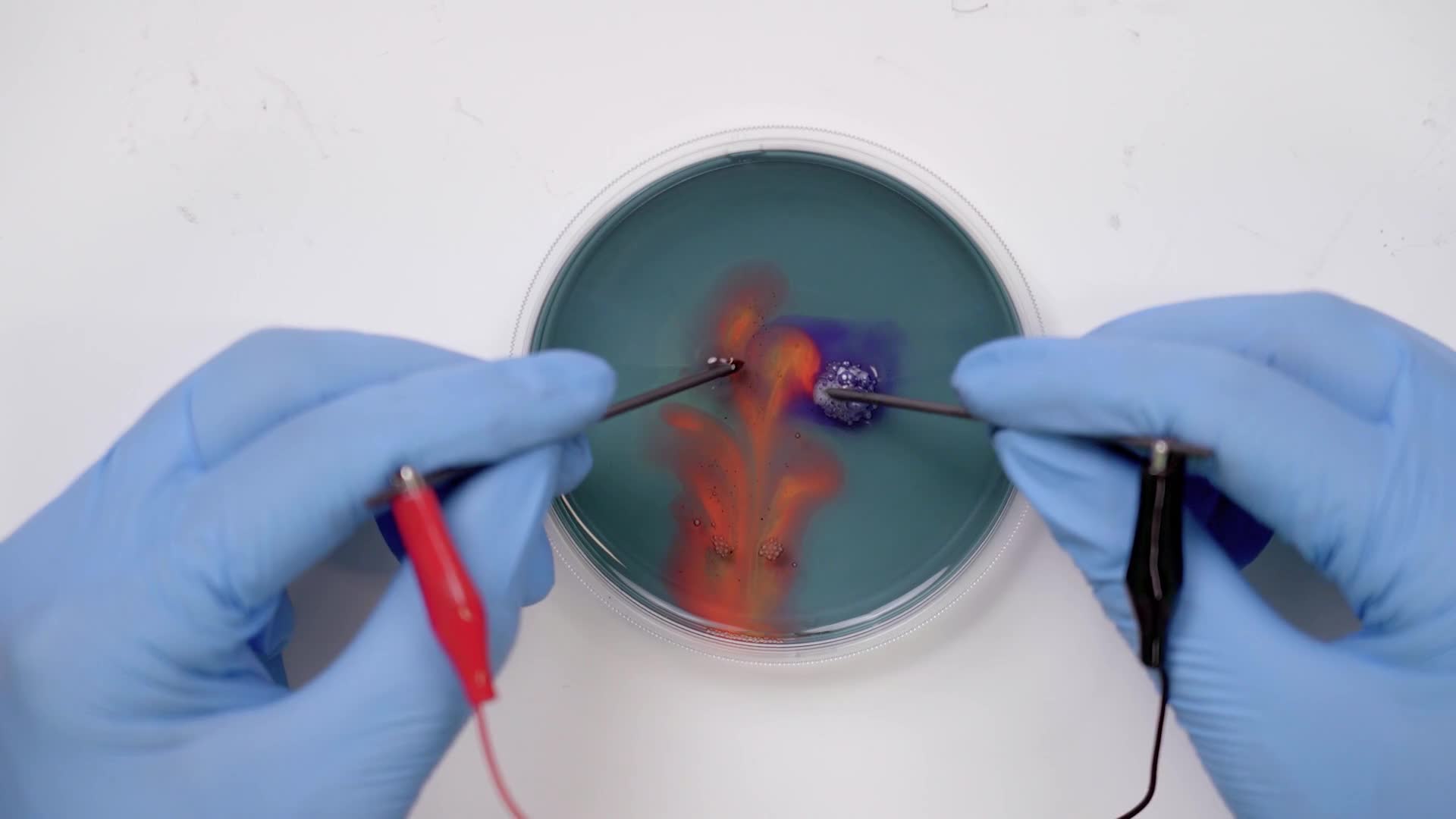
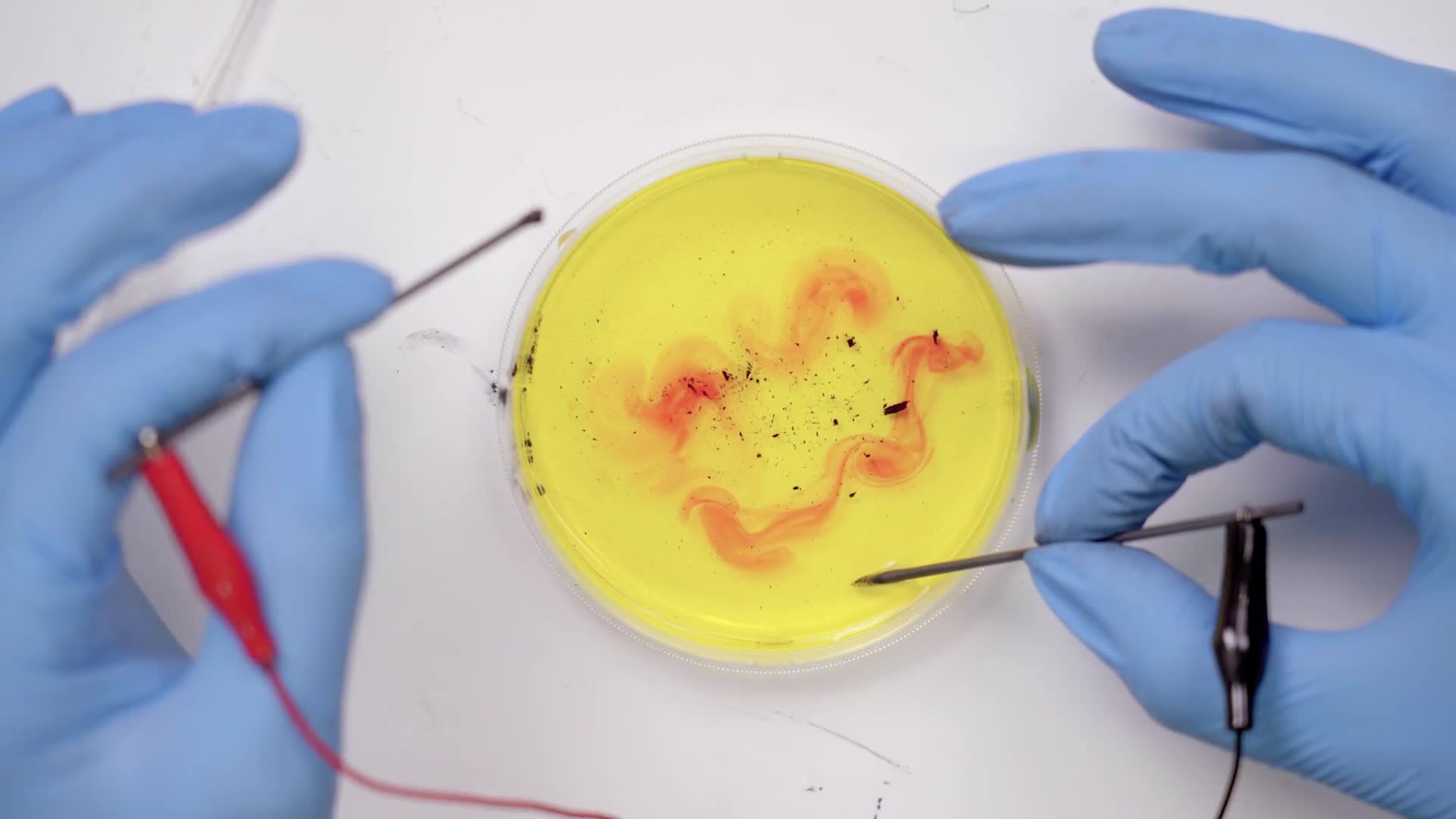
Place the tips of the lead sticks into the petri dish and hold them there for 15 seconds while the reaction starts to happen.

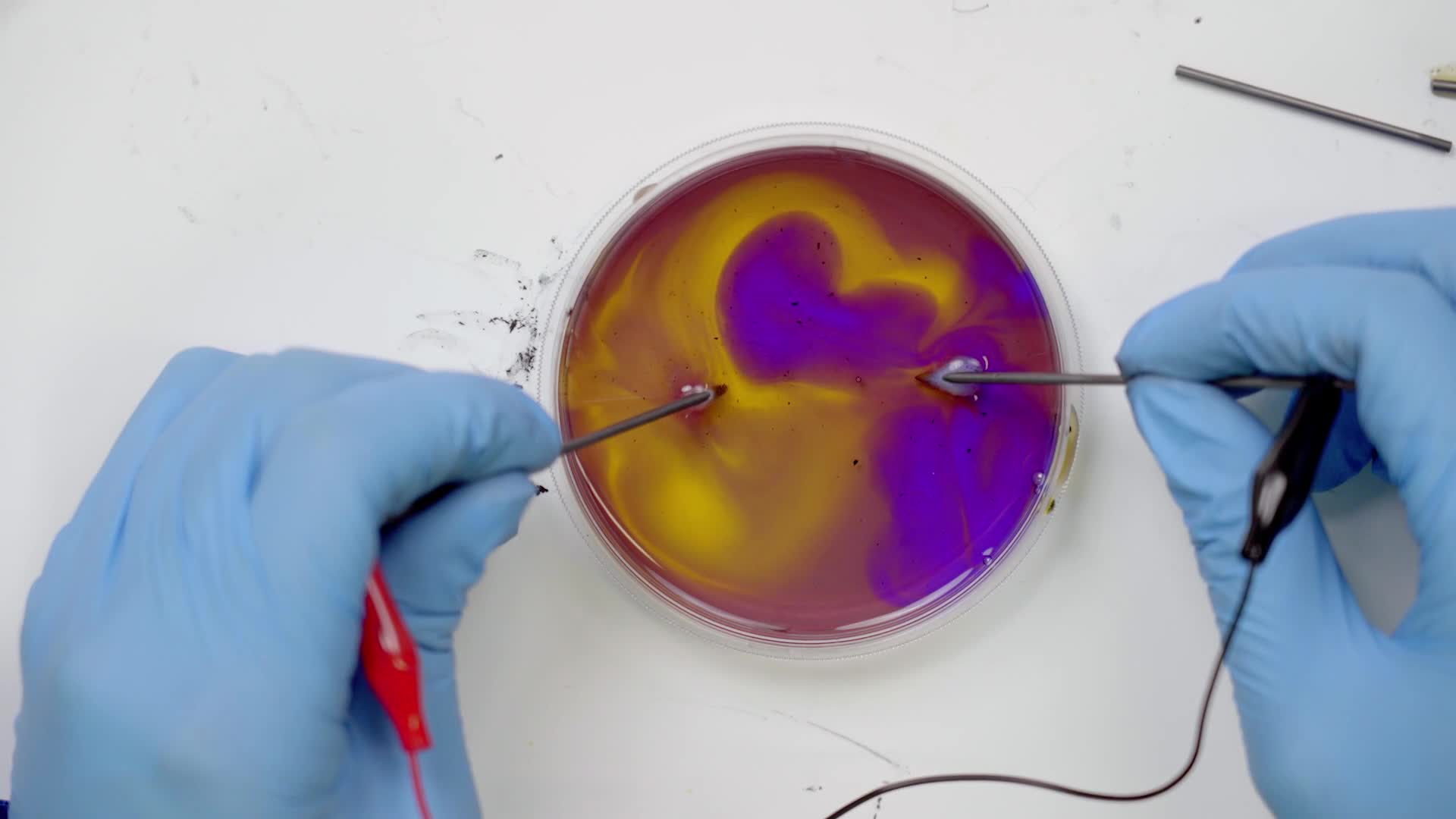
Try moving the sticks around in different directions, like circles. Experiment with all kinds of motions.
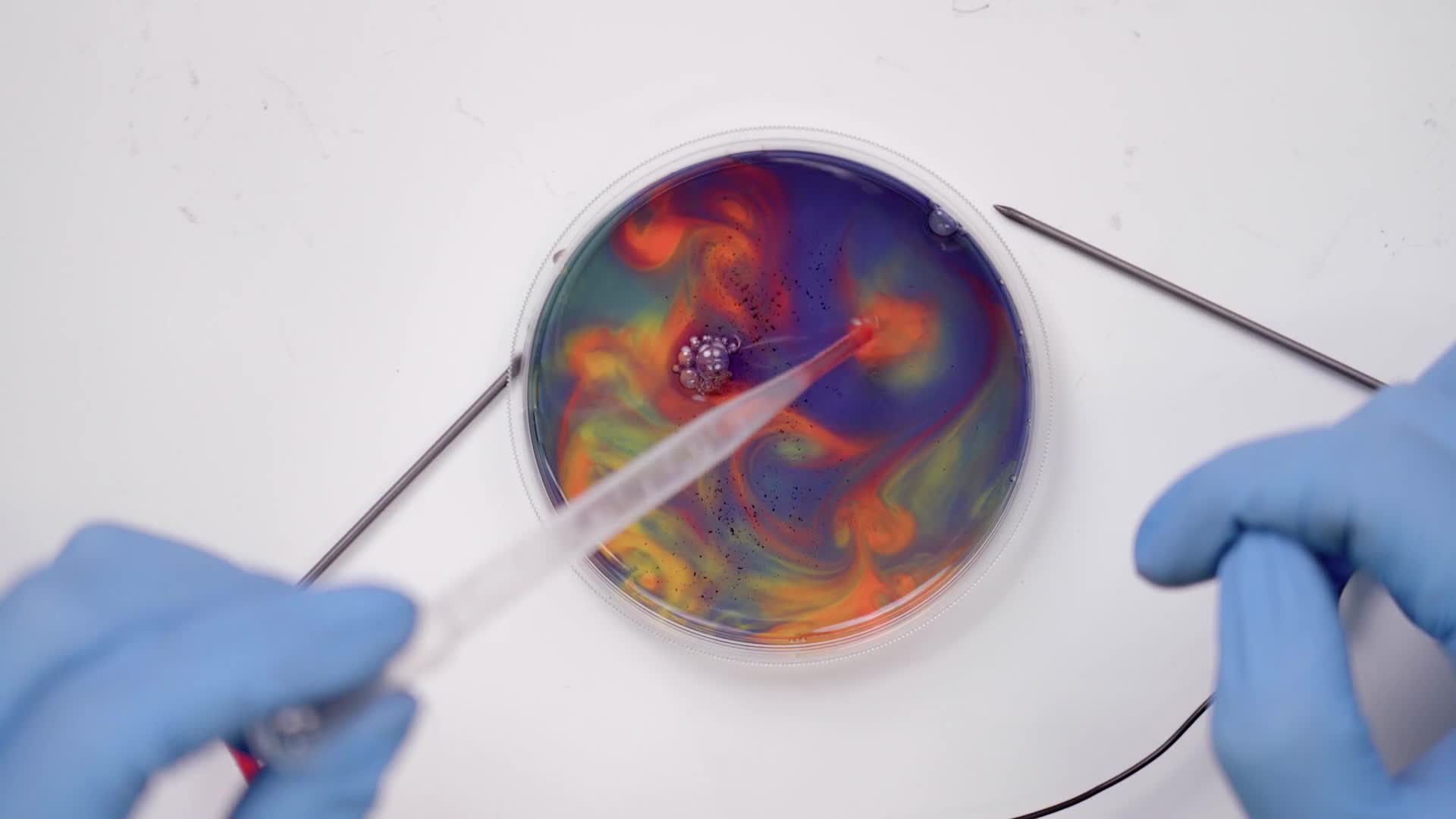
Use the pipette to suck up one color and squeeze it back out into another color to mix them. The pipette can also be used to swirl the colors.
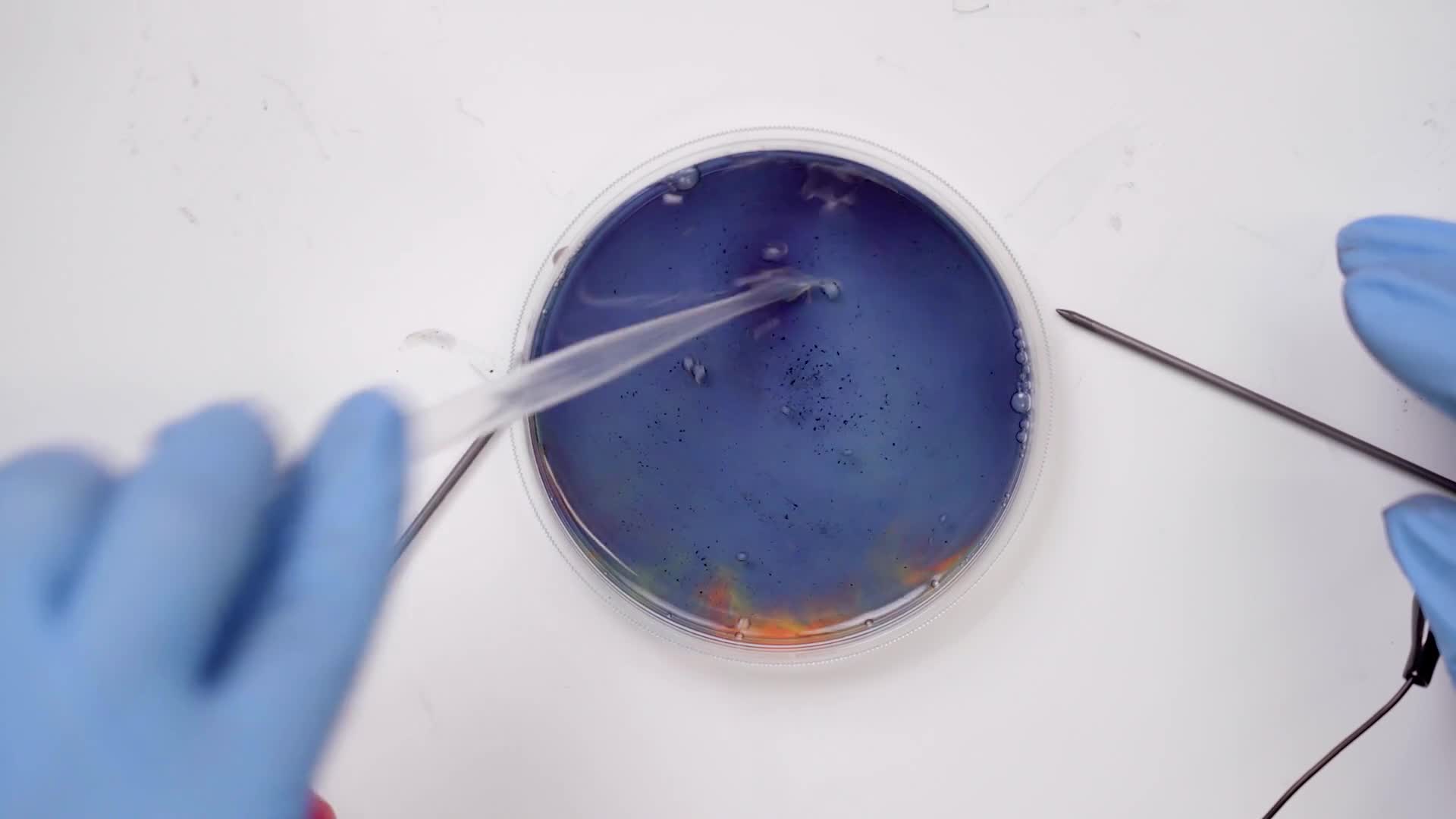
Change the colors back to the original blue color by swirling the pipette around until all the rainbow colors have disappeared.

Squeeze the liquid from the bottle into the petri dish. Remember to keep a little bit in the bottle for later.
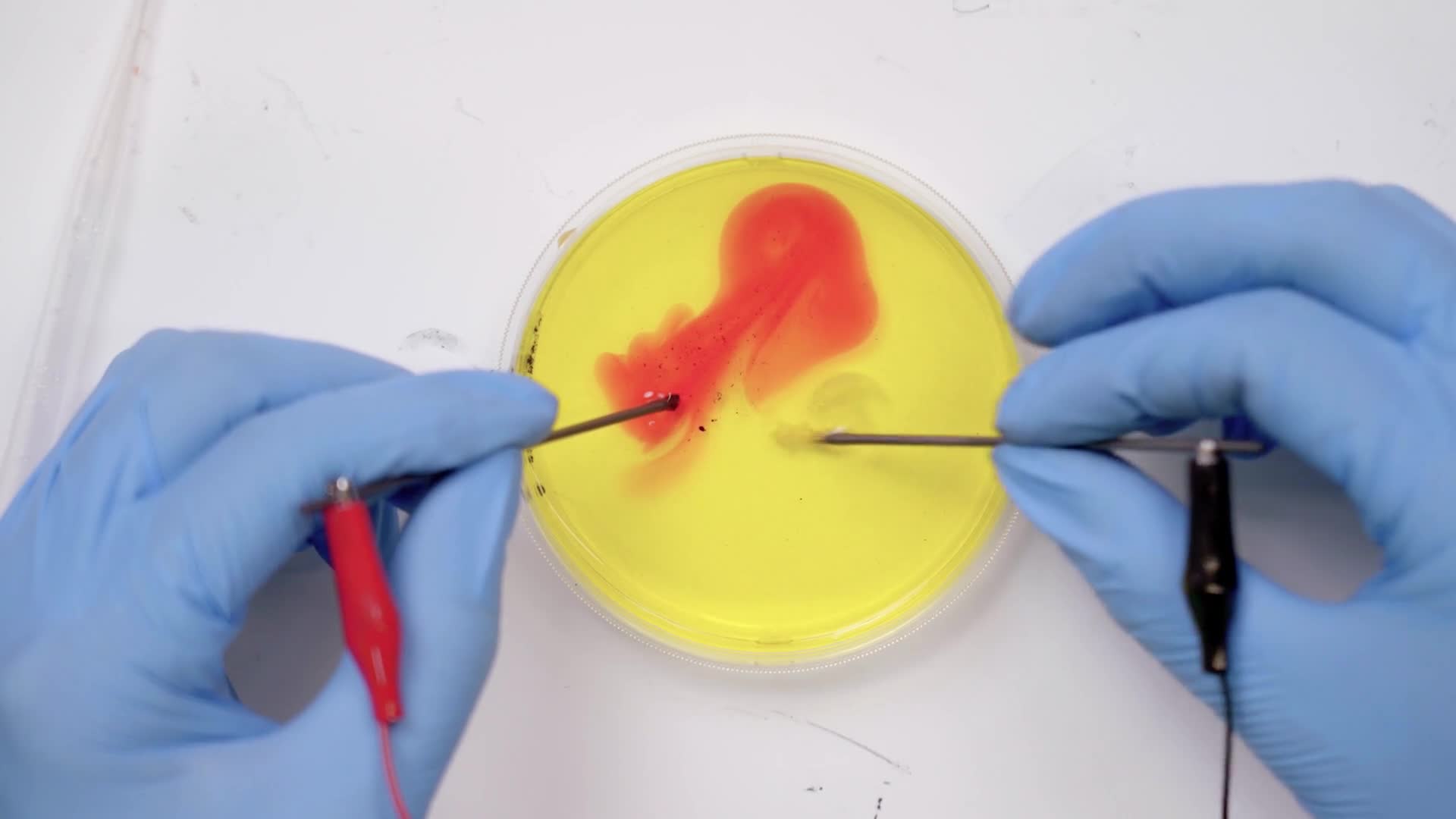
Place your lead sticks into the liquid and wait about 15 seconds before you start moving them around.

When you’re done, gently empty the liquid into the plastic cup and setup again to try the final color.

Squeeze some of the liquid into your petri dish and save a little bit for later. Your color may look a little different than mine, but that’s okay!
How It Works
We can see hydrogen gas and oxygen gas bubbling off of those black rods. Now that splitting process changes the property of the water near those rods. On one side it becomes acidic like lemon juice and on the other side, basic like baking soda. But if that’s all that was happening it would be clear and we would just see bubbles. We used another chemical called an indicator to indicate to us that the property of the water is changing by changing the colors.
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
We use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More