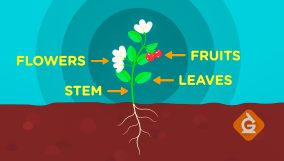
Thermometer Definition
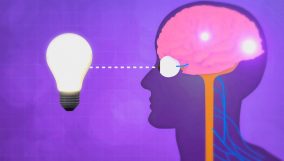
A thermometer measures a substance's temperature by its particles' speed. For example, it indicates how hot or cold water is.
View Lesson on Intro to Thermal Energy
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Intro to Thermal Energy
Fun Facts
- A thermometer measures an average temperature because the particles within a substance do not all move at exactly the same speed.
- A thermometer shows that the temperature of ice is 0 degrees Celsius, the temperature of steam is 100 degrees Celsius, and the temperature of liquid water is in between.
- A surface thermometer uses an infrared or liquid crystal strip.
Why Do We Need To Know About Thermometer
Learning about thermal energy helps you understand how heat changes things and why thermometers are important in many areas. For example, in cooking, knowing the right temperature makes sure food is safe and tastes good. This is important for baking cookies or heating food in microwaves.
Thermometers are also key in different jobs like environmental engineering, the military, and healthcare. They help in making fake snow, heating ready-to-eat meals, or using cold packs in sports. So, measuring and managing heat is useful in lots of careers and everyday life.
Frequently Asked Questions
Check out the Full Lesson on Intro to Thermal Energy
In this lesson, we learn that:
- All atoms are constantly vibrating because they have thermal energy.
- Adding or removing thermal energy can change the state of matter.
- Chemical reactions can release or absorb thermal energy.
Related Topics
- Absorbency Definition
- Algae Definition
- Biosphere Definition
- Biotechnology Definition
- Body Fossils Definition
- Computer Programming Definition
- Conservation Biologist Definition
- Continental Drift Definition
- Decomposer Definition
- Definition Of Nutrients
- Dissolve Definition
- Distillation Definition
- Earth’s Rotation Definition
- Electricity Definition
- Fossil Definition
- Genetic Factors Definition
- Geosphere Definition
- Insulator Definition
- Internal Structures Definition
- Kuiper Belt Definition
- Landslide Definition
- Light Source Definition
- Liquid Nitrogen Definition
- Lunar Mare Definition
- Material Definition
- Matter Definition
- Molecule Definition
- Moon Definition
- Natural Resource Definition
- Newton’s 1st Law Of Motion Definition
- Newton’s 2nd Law Of Motion Definition
- Photosynthesis Definition
- Plant Definition
- Predator Definition
- Property Definition
- Proton Definition
- Pull Definition
- Scientist Definition
- Sedimentary Rock Definition
- Sound Definition
- Sound Wave Definition
- Surface Runoff Definition
- Thermal Energy Definition
- Thermometer Definition
- Total Eclipse Definition
- Virus Definition
- Water Erosion Definition
- Weight Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
Use Generation Genius in Your School
Access all lessons free for 30 days.
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


to Discover the Benefits of Generation Genius
Learn How to Save for Your School & District!

no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More