Thermal Energy Definition
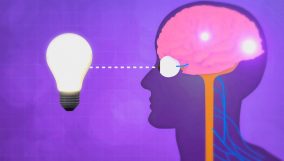
Thermal energy is energy from atoms moving in a substance. For example, ice melting involves thermal energy transfer, raising its temperature.
View Lesson on Intro to Thermal Energy
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Intro to Thermal Energy
Fun Facts
- Chemical reactions, like fire, can release or absorb thermal energy.
- Thermal energy always move from a hotter area to a colder area.
- Insulators like cardboard and foam can reduce the amount of thermal energy that is transferred into or out of a substance.
Why Do We Need To Know About Thermal Energy
Learning about thermal energy helps us understand how we cook food and keep warm. It’s all about moving heat around. This is important for staying comfortable and alive. For example, when we bake cookies or use hand warmers, it’s thermal energy that makes things hot or changes their state.
This knowledge can also help you find jobs in areas like food science, working with the army, or designing new products. Knowing about thermal energy means you can come up with better ways to keep things warm or cold, like in a thermos, or make fake snow, showing it’s useful for a lot of different things.
Frequently Asked Questions
Check out the Full Lesson on Intro to Thermal Energy
In this lesson, we learn that:
- All atoms are constantly vibrating because they have thermal energy.
- Adding or removing thermal energy can change the state of matter.
- Chemical reactions can release or absorb thermal energy.
Related Topics
- Air Mass Definition
- Batteries Definition
- Binary Code Definition
- Bioindicator Definition
- Carbon Dioxide Definition
- Cellular Respiration Definition
- Competition Definition
- Computer Programming Definition
- Conservation Biologist Definition
- Definition Of Engineering
- Definition Of Living Things
- Definition Of Nutrients
- Ecosphere Definition
- Electron Definition
- Gas Definition
- Germination Definition
- Hydrosphere Definition
- Invasive Species Definition
- Landform Definition
- Lever Definition
- Life Cycle Definition
- Light Reflection Definition
- Marsupial Definition
- Metamorphosis Definition
- Multicellular Definition
- Nervous System Definition
- Newton’s 1st Law Of Motion Definition
- Ocean Current Definition
- Opposable Thumb Definition
- Orbit Definition
- Partial Eclipse Definition
- Property Definition
- Recycle Definition
- Respiratory System Definition
- River Definition
- Seed Dispersal Definition
- Seeing Definition
- Senses Definition
- Signal Definition
- Soil Definition
- Solar System Definition
- Solid Definition
- Taxonomy Definition
- Temperature Definition
- Thermal Energy Definition
- Thermometer Definition
- Translucent Definition
- Unbalanced Force Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More