Heat Definition
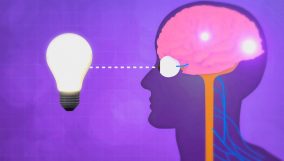
Heat is the transfer of thermal energy from one object or region to another. For example, sunlight increases the temperature of a surface.
View Lesson on Heat: Transfer of Thermal Energy
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
Heat: Transfer of Thermal Energy
Fun Facts
- A hot cup of coffee transfers heat to the surrounding air over time.
- A thinner copper rod melts chocolate faster than a thicker one when heated.
- Heated fluid rises, but liquids are not very good conductors of heat.
Why Do We Need To Know About Heat
Learning about how heat moves helps us know why certain things, like jackets or emergency blankets, are made the way they are. This keeps us warm, safe, and saves energy. For example, how a jacket keeps you warm or how a shiny emergency blanket stops heat from escaping shows how important it is to control heat in making products.
Also, things like solar cookers, which use the sun to cook food, and how computers stay cool show us the role of heat in using clean energy and in gadgets. Knowing about heat transfer opens up job chances in areas like heating and cooling systems, making appliances, and ensuring fire safety. This shows how knowing about heat is useful in many different jobs.
Frequently Asked Questions
Check out the Full Lesson on Heat: Transfer of Thermal Energy
In this lesson, we learn that:
- Heat is the transfer of thermal energy from one object to another.
- Heating can occur by conduction, convection and radiation.
- Some materials can store more thermal energy than others.
Related Topics
- Adaptation Definition
- Batteries Definition
- Body Fossils Definition
- Cell Definition
- Chromosome Definition
- Circuit Definition
- Circulatory System Definition
- Conduction Definition
- Convection Definition
- Decomposer Definition
- Definition Of Engineering
- Earth’s Axis Definition
- Earth’s Orbit Definition
- Electromagnet Definition
- Element Definition
- Energy Definition
- Energy Transfer Definition
- Evaporation Definition
- Gas Definition
- Gas Definition
- Greenhouse Effect Definition
- Group Behavior Definition
- Heat Definition
- Hydrosphere Definition
- Larvae Definition
- Lever Definition
- Marsupial Definition
- Mutation Definition
- Mutualism Definition
- Ocean Current Definition
- Organ Definition
- Organelle Definition
- Property Definition
- Renewable Energy Definition
- Reversible Change Definition
- Sediment Filter Definition
- Seed Dispersal Definition
- Solar System Definition
- Sound Definition
- Temperature Definition
- Total Eclipse Definition
- Transverse Wave Definition
- Vibrating Definition
- Water Erosion Definition
- Water Quality Definition
- Watershed Definition
- Wave Reflection Definition
- Wedge Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
Use Generation Genius in Your School
Access all lessons free for 30 days.
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


to Discover the Benefits of Generation Genius
Learn How to Save for Your School & District!

no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More