Electromagnetic Radiation Definition
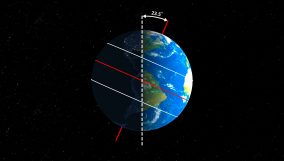
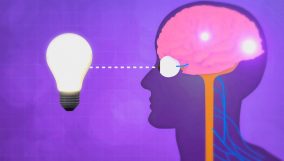
Electromagnetic radiation transfers energy via waves, varying from radio waves to gamma rays. For example, visible light is a type of electromagnetic radiation.
View Lesson on Electromagnetic Spectrum
Become a member to get full access to our entire library of learning videos, reading material, quiz games, simple DIY activities & more.
Become a member to get full access to our entire library of learning videos, quiz games, & more.
Plans & Pricingto watch this full video.

Access All Videos
and Lessons, No Limits.
Access All Videos

No credit card required,
takes 7 sec to signup.
No card required

Ready-to-go lessons
that save you time.
Ready-to-go lessons
If you are on a school computer or network, ask your tech person to whitelist these URLs:
*.wistia.com, fast.wistia.com, fast.wistia.net, embedwistia-a.akamaihd.net
Sometimes a simple refresh solves this issue. If you need further help, contact us.
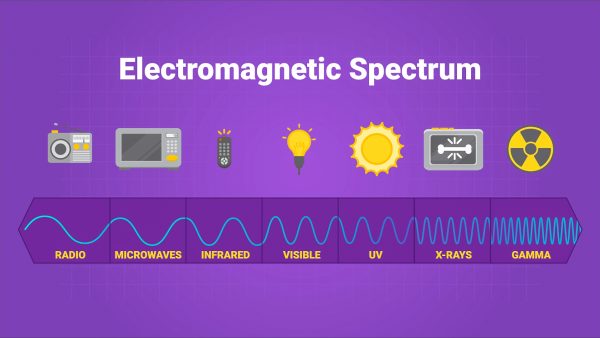
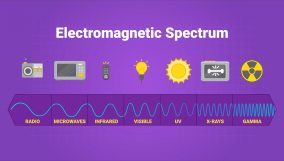
Electromagnetic Spectrum
Fun Facts
- Electromagnetic radiation can travel through empty space.
- Not all forms of electromagnetic radiation are harmful.
- Electromagnetic radiation other than visible light can be used to view an object. For example, bees use ultraviolet waves to help them see patterns on flowers.
Why Do We Need To Know About Electromagnetic Radiation
Learning about electromagnetic radiation helps us understand how things work around us and how important it is for our daily lives. For example, we use it for seeing (light), talking on the phone or watching TV (radio waves), cooking (microwaves), staying warm (infrared), and getting sunlight (ultraviolet). It’s not just about staying alive; it also makes our lives better through new tech and health care.
Also, many jobs in science and technology need a good understanding of electromagnetic radiation. For example, radiologists are doctors who use X-rays to look at your bones and help fix them. Astronomers, who study stars and space, use telescopes that can catch radio waves to watch stars explode. These jobs show how electromagnetic waves are key in creating new things and solving problems.
Frequently Asked Questions
Check out the Full Lesson on Electromagnetic Spectrum
In this lesson, we learn that:
- Electromagnetic radiation is a type of wave that transfers energy.
- It includes radio waves, microwaves, infrared waves, visible light, UV light, X-rays, and gamma rays.
- The difference between all of these is the wavelength of the radiation.
Related Topics
- Carbon Dioxide Definition
- Cast Fossils Definition
- Cell Definition
- Chemical Change Definition
- Chemical Reaction Definition
- Climate Definition
- Comparative Anatomy Definition
- Computer Programming Definition
- Condensation Definition
- Conduction Definition
- Corona Definition
- Decomposer Definition
- Definition Of Nutrients
- Ecosphere Definition
- Electromagnetic Radiation Definition
- Electromagnetic Spectrum Definition
- Evaporation Definition
- Force Definition
- Fossil Record Definition
- Freezing Definition
- Frequency Definition
- Friction Definition
- Habitat Definition
- Hydrosphere Definition
- Inherited Traits Definition
- Invasive Species Definition
- Landform Definition
- Magnetism Definition
- Mass Definition
- Mitochondria Definition
- Moon Definition
- Mutualism Definition
- Natural Disaster Definition
- Natural Resource Definition
- Opposable Thumb Definition
- Orbit Definition
- Paleontologist Definition
- Partial Eclipse Definition
- Particle Model Of Matter Definition
- Potential Energy Definition
- Properties Of Matter Definition
- Solar Eclipse Definition
- Solid Definition
- Taxonomy Definition
- Tsunami Definition
- Water Cycle Definition
- Weather Definition
- Weathering Definition
Start a Free Trial Today. Get a $5 Amazon Gift Card!
Teachers! Start a free trial & we'll send your gift card within 1 day. Only cards left. Try it now.
Select Grade
Select Subject
This email is associated with a Science Kit subscription. Kit subscriptions are managed on this separate page: Manage Subscription

-
Download InvoiceScience & Math$/yr
-
Download InvoiceScience Only$/yr

access all lessons
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
"My students loved the videos. I started the video subscription in May and used them as a review before the state test, which I know contributed to 100% of my class passing the state test."
Rhonda Fox 4th Grade Teacher, Ocala, Florida
• No credit card required •
Already a member? Sign In
* no credit card required *

* no credit card required *
* no credit card required *


no credit card required
Skip, I will use a 3 day free trial
Enjoy your free 30 days trial
-
Unlimited access to our full library
of videos & lessons for grades K-5. -
You won’t be billed unless you keep your
account open past your 14-day free trial. -
You can cancel anytime in 1 click on the
manage account page or by emailing us.
-
Unlimited access to our full library of videos & lessons for grades K-5.
-
You won't be billed unless you keep your account open past 14 days.
-
You can cancel anytime in 1-click on the manage account page.
Cancel anytime in 1-click on the manage account page before the trial ends and you won't be charged.
Otherwise you will pay just $10 CAD/month for the service as long as your account is open.
Cancel anytime on the manage account page in 1-click and you won't be charged.
Otherwise you will pay $10 CAD/month for the service as long as your account is open.
We just sent you a confirmation email. Enjoy!
DoneWe use cookies to make your experience with this site better. By using this site you agree to our use of cookies. Click "Decline" to delete and block any non-essential cookies for this site on this specific property, device, and browser. Please read our privacy policy for more information on the cookies we use.Learn More
We use cookies to improve your experience. By using this site, you agree to our use of cookies. Click "Decline" to block non-essential cookies. See our privacy policy for details.Learn More